You can download our product and service brochures here for easy access.
Our technical expert team has assembled a list of frequently asked questions to help you find answers quickly.
What are genetically engineered mice?
Genetically Engineered Mouse Models are mice of specific phenotypes or characteristics that are obtained by artificial modification of mouse genome via genetic engineering techniques. They are the most important animal models for studying gene functions and human diseases. Common genetically engineered mouse models include gene knockout, conditional gene knockout, gene knock-in (point mutation or fragment knock-in), transgene, and targeted transgene (overexpression). Genetically engineered mouse models can be tailored to your individual needs.
How long does it take to obtain a genetically engineered mouse model listed in the repository?
If the strain has a " Repository live ", it means that the strain has live mice at this moment that can be immediately available or used for breeding. These mice can be provided directly or after breeding upon your request.
If the strain is in the state of " Cryorecovery available", it means that the embryos of this strain need to be resuscitated, which takes 3 months in general.
What documents are required to order custom models of genetically engineered mice?
You only need to provide the name of the genes to be knocked out or engineered and other special requirements.
According to your requirements, we will design a preliminary protocol for custom models. After both parties reach a mutual agreement, a technical service contract will be signed.
You can contact us by:
emailing the gene names and specific requirements to service.us@modelorg.com.
What is AAALAC certification?
The full name of AAALAC is the Association for Assessment and Accreditation of Laboratory Animal Care. AAALAC International certification is a symbol for the quality and biosafety standard of experimental animals. As a sign of quality suitable for internationally leading medical research, AAALAC International certification represents the true commitment to humane care of animals and is a prerequisite for organizations involved in the application and production of experimental animals to participate in international exchanges and competition.
What is SPF?
SPF is the abbreviation of "Specific-pathogen-free" and represents animals which do not carry specific pathogens and parasites that may interfere with the experiment. SPF animals should be housed in a barrier system, and are subject to strict microbial and parasite control. As internationally recognized and standard laboratory animals, SPF grade animals can be widely used in life science and medical research experiments.
What is an inbred strain?
An animal strain established by 20 (or more) consecutive generations of mating among siblings (or via mating between a parental generation and a progeny generation). The inbreeding coefficient of the stain should be greater than 99%, and all individuals in the strain should be traced back to a common pair of ancestors.
Characteristics: The genetic background is uniform and the experimental results are consistent. However, due to inbreeding depression and low viability, an inbred strain has a low number of litters and a more stringent demand for the breeding environment and nutritions.
The commonly used C57BL/6, Balb/c, and FVB all belong to inbred strains.
How many pairs of mice are generally needed for strain breeding?
In general, at least 2–4 pairs of mice at the breeding age are required for strain breeding. If the mice of a special strain have birth defects or require accelerated breeding, consider increasing the number of mating pairs. Alternatively, an IVF rapid breeding method can be used. In this case, only 2–3 fertile male mice are needed.
What measures to take if the mice are having difficulty breeding?
Check for the following problems when the number of offspring is decreased in the breeding process or when no offspring is born:
The breeding environment is too noisy: during the breeding and feeding of mice, noise should be minimized to ensure that the mice live in a quiet environment.
Pregnant mice are stimulated by external stress: The operation and environmental changes incurred to pregnant mice should be minimized. When pregnant mice and lactating mice are stimulated by external stress, they may eat or abandon their cubs.
The mating time between male and female mice is too short: Ensure an appropriate length of mating time between male and female mice to increase the chance of mating. In addition, do not remove mating male mice from the cage when pregnant mice are in labor, and do not put the male mice back into the breeding cage before the offspring mice are weaned.
Increase the darkness of the environment: avoid unnecessary light in the night rhythm because it can affect the circadian rhythm of the mice.
The mice are unable to build nests: Provide soft fiber materials, such as cotton wool, paper towels or cork flocculus, in cages to facilitate the establishment of a safe and comfortable environment for labor, thus increasing the number of litters.
Nutritional deficiency: If the lipid component is too high or too low in mouse diet, or if the protein intake is insufficient or excessive, the reproduction of mice will be affected.In addition, the source of tocopherol, such as seeds and nuts, can be provided as appropriate.
What is the reason to use sentinel mice?
Sentinel mice mainly refer to a type of mice with a healthy background that are introduced into important breeding groups. Through direct contact with the target mice or indirectly contact with dirty litter, the inspection of pathogenic microorganisms in the breeding area can be tested using sentinel mice. In addition, sentinel mice can be used to substitute important breeding groups to undergo medical examinations.
In general, healthy mice (excluding immunodeficient mice) that are older than 4 weeks and not contaminated by specific pathogens can be selected as sentinel mice. The strains of sentinel mice, such as ICR (CD1), BALB/c, FVB, and C57BL/6, can be selected as needed. Sentinel mice are usually housed at a density of 2–3 mice per cage. In addition, they are placed in the lowest position on the cage shelf. Typically, a cage of sentinel animals is used to monitor case shelves housing 60–100 cages. The litter used for the sentinel mice is the dirty litter that has been used by other mice. About 30–50 g litter is placed in each cage and the sentinel mice must live on the dirty litter for at least 4 weeks.
In general, the national standard stipulates that three months is counted as a testing cycle. Through the examination of sentinel mice, the condition of pathogens in the environment can be monitored in real time without affecting the experimental animals, thus ensuring standardized health and quality of experimental animals as well as repeatable experimental results. The examination of sentinel animals also has its limitations. For example, airborne pathogenic microorganisms not transmitted by contact may be overlooked, such as Sendai virus and Pasteurella pneumophila. Therefore, it is recommended to also examine random animals.
How do I establish a strain after the transgenic Founder mice are obtained?
After the Founder mice are obtained, a transgenic strain can be established according to the following procedure.
Each Founder mouse is respectively mated with a wild-type mouse (such as C57BL/6J)
The mating among Founder mice is prohibited
Without a well-defined method for the identification of homozygous and heterozygous mice, the mating among heterozygous mice is prohibited.
It is recommended to mate the positive mice in each generation with wild-type mice, and it is not recommended to use the wild-type mice from the same litter
It is recommended to mate each positive mouse in the F1 generation with wild-type mice until the F2 generation or above is obtained. The experiment can be initialized after the expression of transgene is identified.
Notes about transgenes mediated by the Piggybac transposase system:
The Piggybac transposase tends to insert the target fragment into a transcriptionally active region, thus greatly increasing the probability of obtaining Founder mice with positive expression of the target gene. However, the insertion of transgenes mediated by the Piggybac transposase system may cause gene integration at multiple sites.Due to the existence of integration at multiple sites, the following phenomena will occur during the subsequent breeding of Founder mice along with the separation of multiple integration sites: 1. The proportion of mice with positive results of genotype identification is higher than that of single site insertion; 2. In progeny mice with positive expression of the target gene, the level of expression is lower than that in the Founder mice (the amount of reduction is dependent on the number and location of integration sites in Founder mice) and is inconsistent among different progeny mice.Therefore, it is recommended that each Founder mouse be backcrossed with wild-type mice for at least 2–3 generations to obtain transgenic mice with single site integration and stable expression of the exogenous gene.
How do I establish a strain after the transgenic Founder mice are obtained?
After the Founder mice are obtained, a transgenic strain can be established according to the following procedure.
Each Founder mouse is respectively mated with a wild-type mouse (such as C57BL/6J)
The mating among Founder mice is prohibited
Without a well-defined method for the identification of homozygous and heterozygous mice, the mating among heterozygous mice is prohibited.
It is recommended to mate the positive mice in each generation with wild-type mice, and it is not recommended to use the wild-type mice from the same litter
It is recommended to mate each positive mouse in the F1 generation with wild-type mice until the F2 generation or above is obtained. The experiment can be initialized after the expression of transgene is identified.
How do I breed conventional knockout mice or genetically mutated mice? How do I choose control mice for experiments?
For knockout or genetically mutated mice with an inbred background, mating between heterozygous knockout mice is generally carried out to obtain homozygous knockout or genetically mutated mice. At this time, wild-type or heterozygous mice born in the same litter are generally selected as the control group for the experiment.
Removal of neo from knockout mice
If the removal of neo may cause a complete loss of gene functions and if there are no other genes in the vicinity of the genome, the neo gene does not have to be removed. On the other hand, if only a certain functional domain is knocked out, some proteins with partial functions may appear. Alternatively, there may be other genes located around the knockout gene. In these two cases, it is suggested to remove the neo gene to avoid the occurrence of a phenotype not consistent with the gene knockout. If there is sufficient time, it is suggested to remove the neo gene to avoid the occurrence of other phenotypes caused by neo.
How do I breed conditional knockout mice? How do I choose control mice for experiments?
Conditional knockout mice (also known as Flox mice) are mice whose target genes contain paired loxp sites. After mating them with Cre mice, target genes can be knocked out in specific tissues or cells.
Option One
If Flox mice are mated with Cre mice widely expressing Cre or expressing Cre only in germ cells, systemic KO mice can be obtained. The breeding procedure is shown in the figure. In the ES targeting vector, the screening gene neo is located between two loxp sites and it is not necessary to remove neo separately. The structure is 5' arm-loxp-flox-frt-neo-frt-loxp-3' arm and the neo gene can be simultaneously removed by directly mating with mice expressing Cre in the gonad.
Option Two
The procedure shown in Figure 4 can be adopted if breeding is carried out by mating with mice showing tissue-specific Cre expression. In general, heterozygous flox mice transfected with Cre recombinase are mated with heterozygous flox mice to obtain homozygous conditional knockout mice carrying integrated Cre enzyme. At this time, homozygous flox mice in the same litter that do not carry integrated Cre enzyme are generally selected as the control group for the experiment.
Option Three
The protocol in Figure 5 can also be used to increase the proportion of mice in the experimental group.
What is CreERT2?
By fusing the ligand binding region (LBD) of the human estrogen receptor (ER) with Cre recombinase, a fusion protein (Cre-ER) localized in the cytoplasm can be generated. Only upon estrogen induction, the fused Cre protein can dissociate from the anchor protein HSP90 via conformational changes and enter the nucleus, where the Cre protein recognizes the loxP site and undergoes recombination. In this way, by controlling the injection time of estrogen, the time-specific regulation of gene recombination can be achieved.
In order to avoid the interference by endogenous estrogen, a point mutation (G521R) can be generated in the ligand binding region, thus allowing Cre-ER to only respond to the induction by exogenous synthetic estrogens (e.g., Tamoxifen, 4-OHT). Such fusion protein is named as Cre-ERT. Another fusion protein carrying LBD mutation, the well-known Cre-ERT2, was shown to be much more sensitive to 4-OHT than Cre-ERT. It carries three point mutations in the human ER LBD: C400V/M453A/L544A.
Does the Cre strain only show cleavage activity in specific cells or tissues?
Since Cre is driven by a brain-specific promoter, does that mean Cre is only expressed in the brain? This is not true unfortunately. Studies have shown Cre activity in other tissues of most strains. For example, in addition to the brain, Cre is also expressed in the kidneys and thymus of Emx1-Cre mice.
Can the knockout efficiency of all Cre strains be predicted?
Not in all strains. Some Cre-expressing strains may show large variation in terms of Cre expression even in littermates. In addition, caution should be taken for the strains with a mosaic pattern of Cre expression. You may see large experimental variations when using these mice, and hence it is difficult to repeat experimental results. In order to obtain valuable experimental conclusions, it may be necessary to analyze the phenotypes of more mice.
Is the recombination efficiency of Cre the same regardless of whether it is from the paternal or maternal inheritance?
In most of the strains studied so far, Cre expression is not affected by the source of Cre inheritance. However, for some strains, such as the EIIa-Cre strain, the recombination efficiency of Cre is higher when the Cre gene is derived from maternal inheritance.
Does a mouse expressing the Cre gene only have a poor phenotype?
Studies have shown that Cre expression in mouse models may also exert toxic side effects, such as infertility and decreased viability. This is especially true for the Cre homozygous mice of certain strains. An excessively high Cre activity may induce the recombination at unknown loxP sites in the genome, thus triggering knockout or translocation and subsequently affecting mouse survival. In addition, in the Cre transgenic strains obtained by microinjection, the insertion of Cre gene may interfere with the functions of certain important endogenous genes.
How do I identify the genotypes of genetically-engineered mice?
The basic principle for the genotyping of genetically-engineered mice by PCR is to utilize the sequence difference between the genome of genetically-engineered mice and that of wild-type mice. Using the mouse genomic DNA as a template for PCR, different genotypes of genetically-engineered mice can be determined with gel electrophoresis based on the band size of specific products generated by the genotypes.
Why are there no non-specific bands or no band at all during genotyping?
During the PCR of genotyping, genomic DNA is usually extracted from mouse tail tissues by crude extraction. The genomic DNA obtained by this extraction method contains a high amount of impurities, which may affect the efficiency of subsequent PCR. Consider improving the extraction method to ensure the quality of extracted genomic DNA. In the PCR system, the concentration of genomic DNA can also affect the efficiency of PCR. Too much or too little genomic DNA can lead to the failure of PCR amplification. Adjusting the PCR system might be a solution. If PCR shows non-specific bands, consider increasing the annealing temperature to enhance the specific binding of the primers.
How do I design primers for genotyping of conventional knockout mice?
Refer to the following figure for primer design.
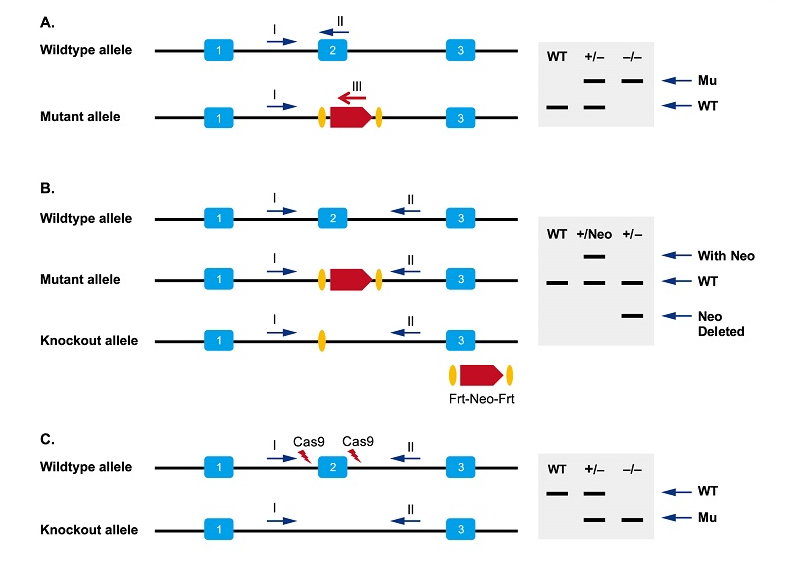
Three-primers genotyping protocol based on ESC targeting; B. Two-primers genotyping protocol based on ESC targeting; C. genotyping protocol based on CRISPR/Cas9.
How do I design primers for genotyping of conditional knockout mice?
Refer to the following figure for primer design.
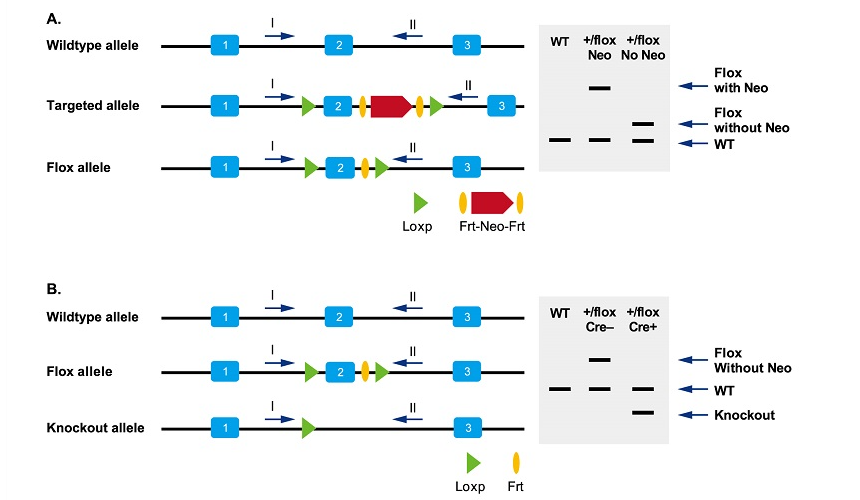
To identify whether the Neo gene in Flox mice has been removed; B. To identify whether the target gene in specific tissues has been knocked out.
Genotyping method for CRISPR/Cas9 Knockout mouse
For knockout mice obtained by random repair via NHEJ, their genotypes can generally be determined by PCR+ sequencing.See the following figure for details:

A.PCR products sequencing chromatogram of wild-type mouse. B. PCR products sequencing chromatogram of F0 generation positive mice. After the action of CRISPR/Cas9, a number of different genotypes can be generated due to repair by NHEJ, thus producing noise peaks near the digestion point of Cas9. C. PCR products sequencing chromatogram of F1 generation positive mice. In the genome of F1 generation positive mice, one copy is a wild type and the other copy is a mutant. The sequencing of the region in the vicinity of the Cas9 action site shows two peaks (two peak shapes are produced for the wild type and mutant genotypes, one for each genotype). C' and C" are the results of monoclonal sequencing of PCR products in Figure C. C' is a wild type and C' is a mutant. The comparison of peak shapes reveals that C" loses two bases compared to C'. The peak shapes of C' and C" are consistent with that of C.
Genotyping method for CRISPR/Cas9 point mutation mouse
Genotyping is generally performed by PCR + sequencing methods. See the following figure for details:
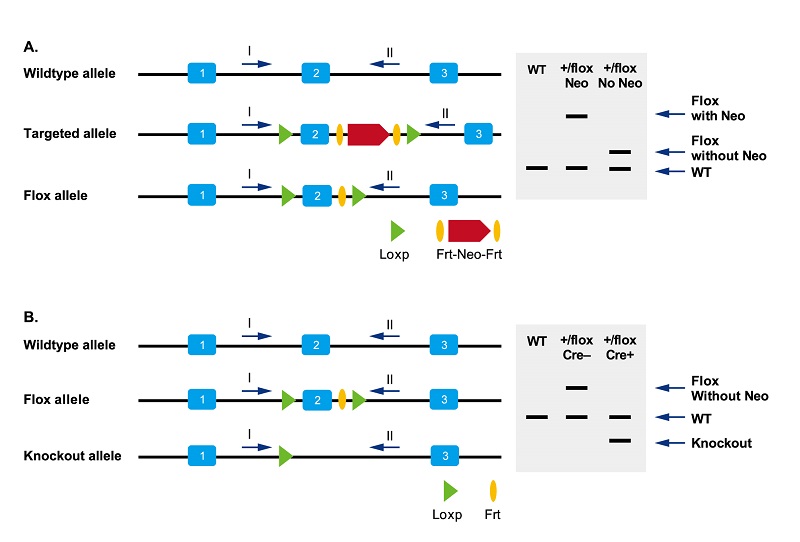
Figure 8. A. To identify whether the Neo gene in Flox mice has been removed; B. To identify whether the target gene in specific tissues has been knocked out.
Shanghai Model Organisms Center (SMOC) provides you with model mice that have been raised under specific pathogen-free (SPF) barriers. Since obtaining AAALAC-accreditation in 2012, Shanghai Model Organisms Center Co., Ltd. has adhered to the standards outlined in the Guide for the Care and Use of Laboratory Animals (Guide). You can download the monthly mice health report here.
SMOC Mice are shipped to international destinations via air transport. Airlines and agents are carefully selected based on their ability to control conditions throughout the journey and their understanding of our animals' unique requirements.
we will arrange to clear the animals through Customs and transport safely to your doorstep. Our Customer Service team will provide an estimate for the cost of this service.
Delivery time cannot be determined. We will determine the transportation time based on the temperature of the place of delivery and the destination.
1. Reduction in body weight
Shipping stress can accelerate the metabolism and excretion of the animals. Without dietary supplements, the animals may display varying levels of dehydration. In general, mice that have been shipped experience weight loss of around 10%. In the event of a long transportation duration or journey, or a high packing density, the mice may experience weight loss of up to 15%. A small number of the animals may even lose up to 30% of their body weight due to factors also directly related to transportation distance, time duration and packing density. Where the aforesaid circumstances arise, the animals can regain any lost weight upon 2–3 days of acclimation and feeding. We recommend that a diet of glucose saline be fed to strains of mice that do not recover easily from dehydration (it is difficult for special strains of mice to recover from dehydration) for a day upon arrival.
2. Increase in experimental error
Laboratory tests have shown that stress may cause a series of pathophysiological changes in the animals, such as adrenal hemorrhage, bleeding, erosion or ulceration in the gastrointestinal mucosa, increase of epinephrine and norepinephrine in the blood, blastoformation inhibition of lymphocytes, and degradation of the toxic function of natural killer cells. These pathophysiological changes may increase certain experimental errors. Therefore, you should try not to conduct the experiment immediately upon receipt of the animals, but acclimate the animals for 2–7 days according to the transportation duration.
According to the relevant provisions on animal transportation in the Regulations for the Administration of Affairs Concerning Experimental Animals, animals of different strains and genders cannot be mixed (which means only animals of the same strain and gender can be placed in the same space).
The standard transportation specifications of the company are as follows:
1. Small air shipping container: single divider insert, two compartments, a maximum of 4 animals in each compartment and 8 ones in a container.
2. Large air shipping container: no divider insert, one compartment, a maximum of 10 male animals
or 15 female animals.
Meanwhile, in the case of multiple animal genotypes, we will divide one shipping container into
several separate compartments by partitioning the space with small cartons, to transport animals
of different strains or genders, for the purpose of lowering your freight charges. (This packing
method is not recommended during the summer).
Mouse Genome Information Database
Please fill in the order form and we will contact you within 2 working days.